Services
Design of Implantable
Medical Devices and Components
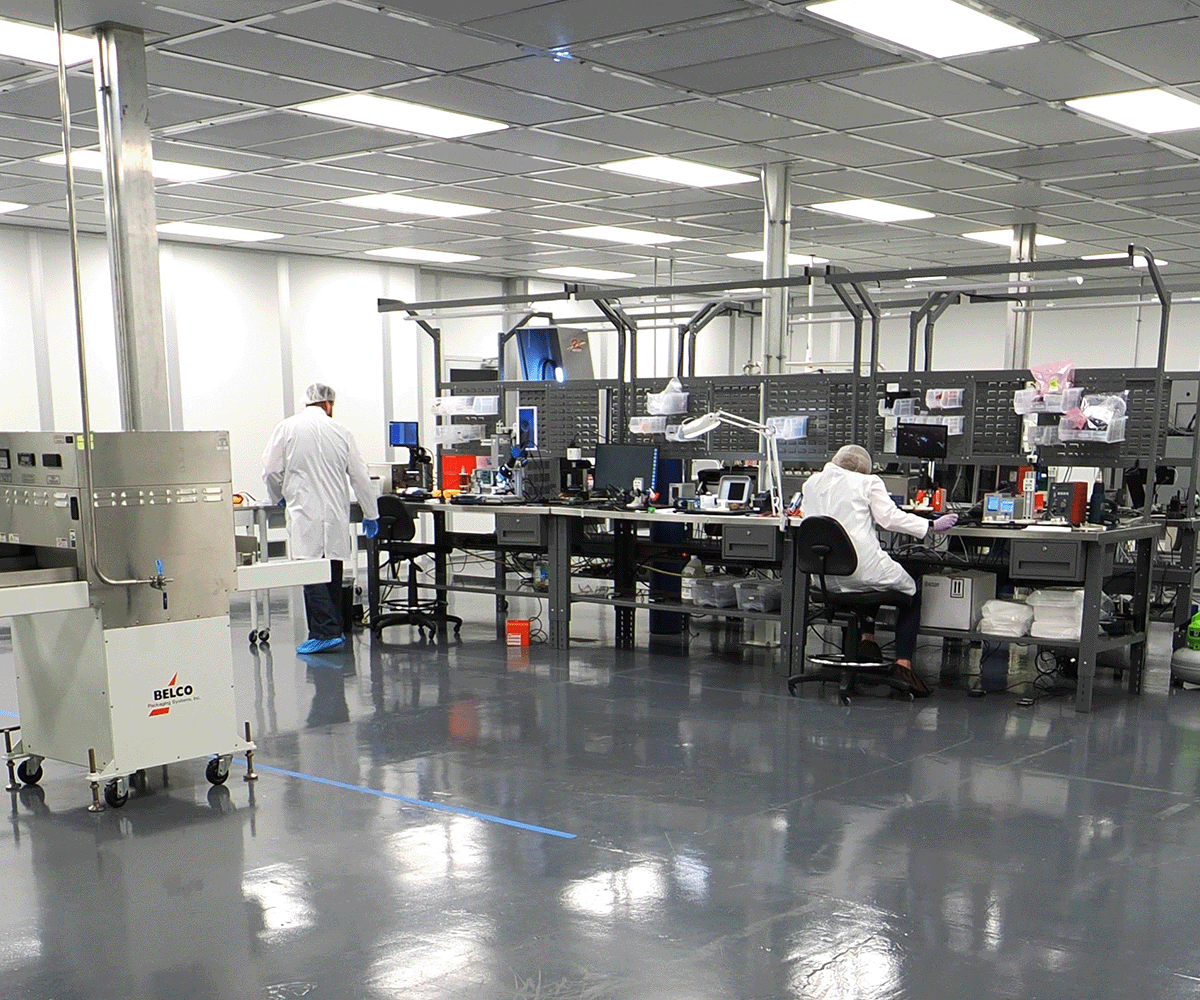
Clients are able to leverage Med-Ally's design and development resources as well as the VersaStim platform to tap into decades of experience in order to mitigate regulatory, engineering, and assembly risks thus speeding up the design process. Med-Ally has contributed to the design of many medical devices for a range of interventional therapies using Implantable Pulse Generators (IPGs) and supporting systems in the areas of Deep Brain Stimulation, Spinal Cord Stimulation, Spinal Cord Injury, Peripheral, Ganglia, Sacral Root, Occipital, Trigeminal, Inter Fascicle and other paradigms.
Design Controls Workflow
New Product Development activity maps the tenants of regimented and methodical Design Controls per applicable section of ISO 13485 with oversight over proper Device Master Record buildout thence submission to the Regulated Agencies. Our workflow, from concept to full scale manufacturing, is a testament of our enforcement and expertise in navigating the complex journey for medical device development, particularly Class III Active Implantable Medical Devices. The graphic below illustrates a brief overview of our device design typical 18 months - or less - Journey
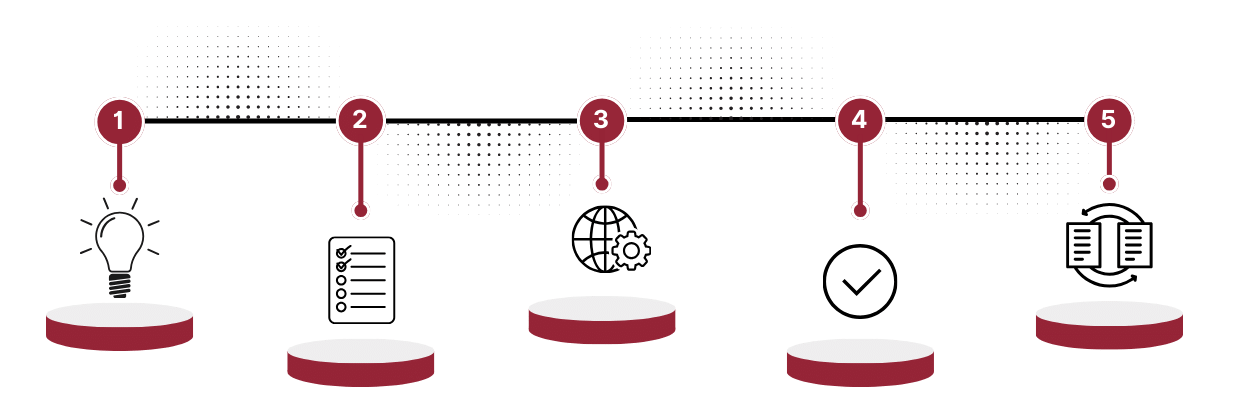
Strategy
Understanding user needs, assessing market opportunities, evaluating core technology, and defining the business model to ensure the medical device meets both clinical and commercial objectives.
Specifics & Planning
Creating a detailed project plan, defining design inputs based on user requirements, reviewing risk management files, and conducting a phase 2 review to ensure all aspects of the device's development are planned and accounted for.
Development
Establishing the architecture and design of the medical device, ensuring that it aligns with the specified requirements and adheres to regulatory standards.
Verification & Validation
Testing the device through verification and validation processes, including labeling and manufacturing evaluation units, to ensure it meets regulatory requirements.
DMR Transfer
Transitions the project from development to manufacturing, involving process qualifications and the transfer of the Device Master Record (DMR), ensuring the device can be manufactured consistently and with high quality.
Prototype Development and Assembly
Our team has instituted novel processes and procedures that effectively provide consistent results to speed up prototype development. We rely on a mix of high-speed and precision robotics to exact hand assemblies and IPC-qualified soldering techniques for exacting prototypes to final products ready for clinical work.
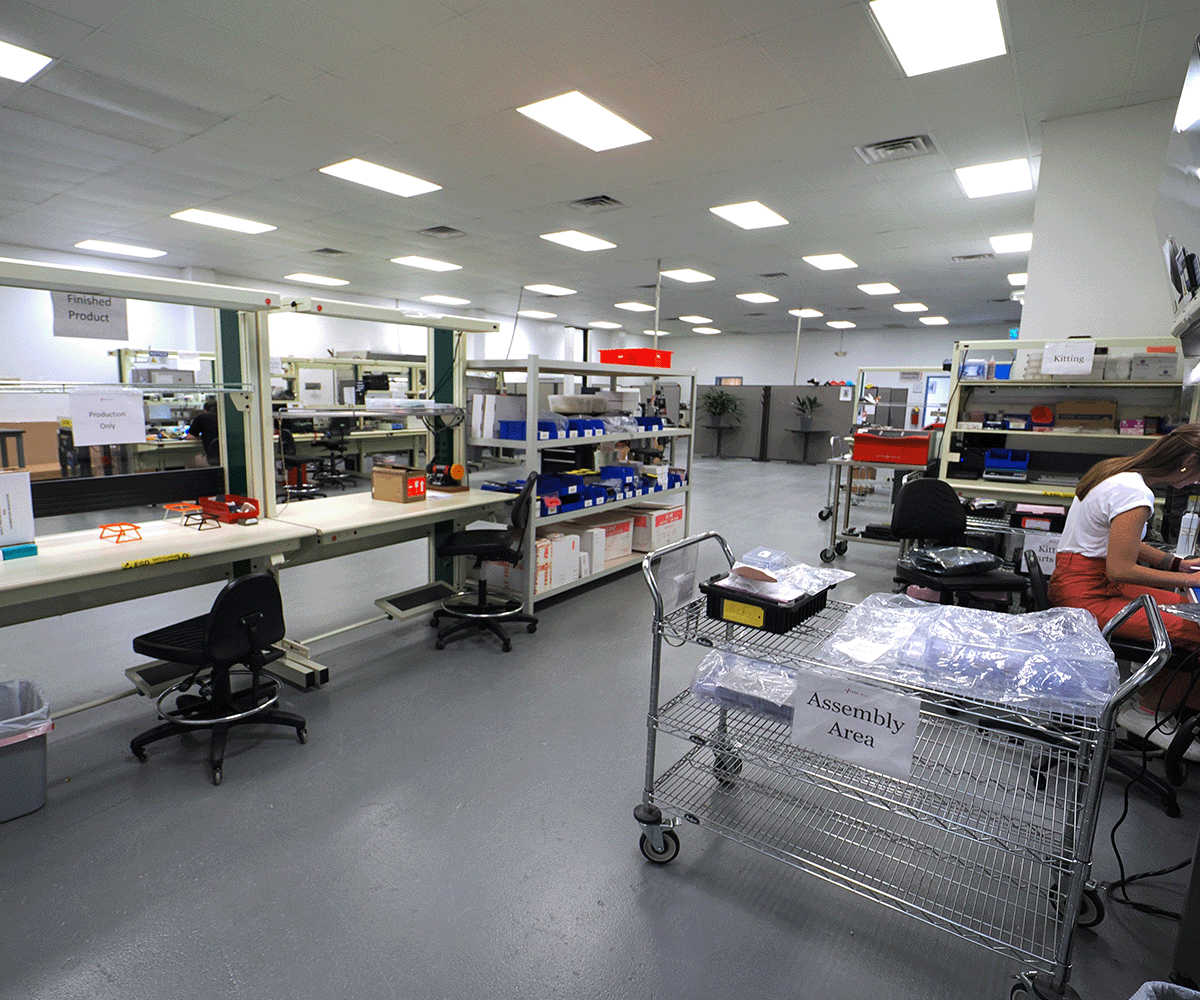
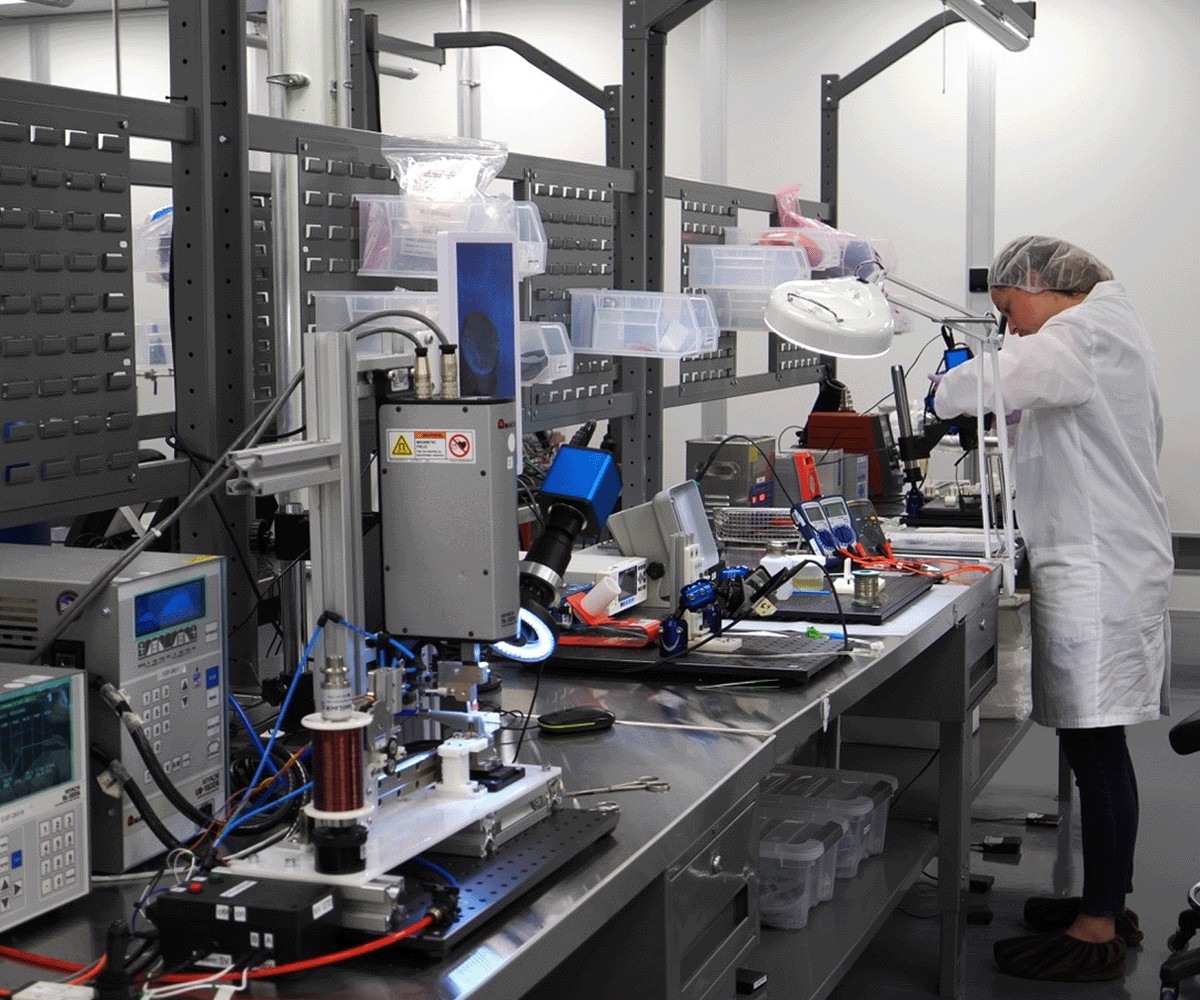
Implanted Medical Device
and Component Manufacturing
The Med-Ally facility is extensively capitalized to provide customers with manufacturing solutions for IPG-based Class III medical implants and controllers. Our commitment to quality and continuous improvement ensures reliable, repeatable outcomes for the highest quality and cost-effective final products. Using a fixed-cost approach, Med-Ally strives to preserve our partnered client’s precious resources for a predictable, budget-friendly pathway using disciplined fiscal management through to development completion.